ß-Lactam Products (penicillins) in Tablets & Capsules Dosage Form

1. Cold Storage Room Preparation and Maintenance
Prior to storing essential RH items like potassium clavulanate, it is important to check that the allocated cold storage space is clean and thoroughly sanitised and that's what is done in the first step. The cold storage chamber is properly checked to ensure that it is kept at the ideal temperature and humidity for preserving the product. By doing this, the stored goods' potency and stability are maintained.
2. Tablet Manufacturing Section Setup
Next, a tablet manufacturing section is set up dedicatedly to handle critical RH products, ensuring it is separate from other manufacturing areas. Appropriate air handling units (AHUs) are installed in the tablet manufacturing section to maintain the required environmental conditions, especially in temperature and humidity. Proceeding with calibration and validating the AHUs regularly is important to ensure their operational requirements can handle seasonal variability, maintaining a stable environment for the manufacturing process.

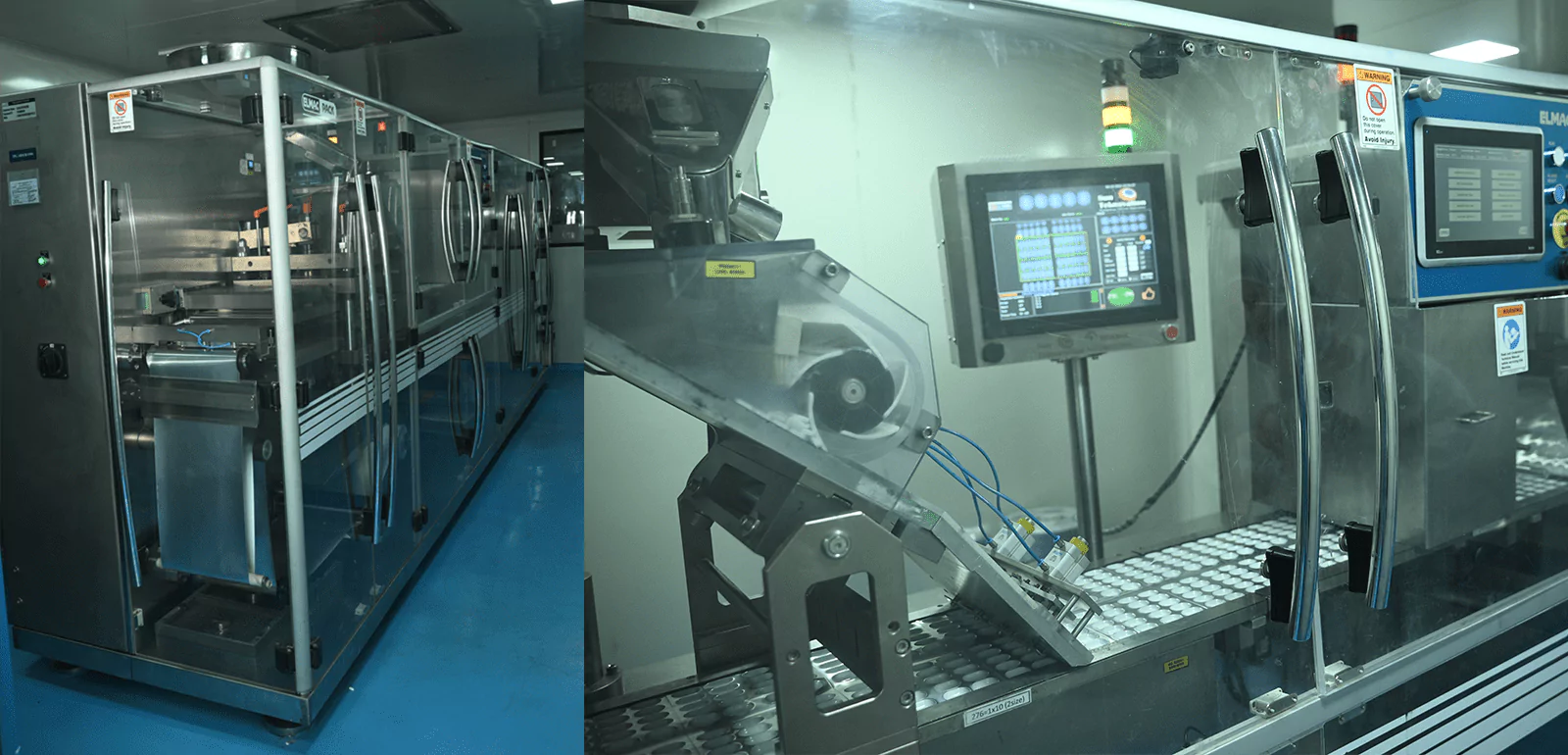
3. Configuration of Capsule Filling Section
The next step is a capsule-filling section with high-capacity compactor machines, double cone and octagonal blenders, and fully automatic AF-40 T and AF-90 T capsule-filling machines. A routine check to ensure that all equipment used in the capsule filling section is appropriately cleaned, maintained, and calibrated to meet the required standards of cleanliness and accuracy is regularly done. Regular training programs for the operators to ensure they are proficient in operating the specific machines and following proper handling procedures for critical RH products are carried along.

4. Dry Syrup Manufacturing Setup
Moving further, After carefully weighing and dispensing the powder blends, it is converted into ready-to-use dry syrup in our futuristic and avant-garde dry syrup manufacturing plant. Equipped with a vibro-motor stainless steel sifter, fluid bed dryer, and a double-conical blender with a rapid mixer granulator. The entire setup also including powder filling, sticker labelling, and carton packing machines is made of food-grade materials providing it with 24-hour operational capacity and up to 90% working humidity. The end product received is a fully packaged and ready-to-dispatch batch of β-Lactam dry syrups.

5. Quality Assurance and Documentation
The last step is compiled with the establishment of comprehensive quality assurance protocols to monitor the entire process of handling critical RH products, from storage to manufacturing and filling. Implementing a robust documentation system to record and track all vital parameters, including temperature, humidity, equipment calibration, and maintenance activities, is followed. Regular quality checks and testing of the final products are done to ensure compliance with the required specifications and regulatory standards.